 |
Combustion Process - Continued.
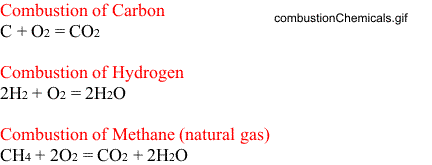
Combustion of Methane (natural gas)
As noted in the above figure, for complete combustion of methane, the following reaction takes place.
One molecule of methane reacts with two molecules of oxygen to yield one molecule of carbon dioxide and two molecules of water.
We can write the same equation in terms of atomic weights:
16 + 64 = 44 + 36 (using atomic weight of entities)
Dividing all quantities by 16 we get:
1 + 4 = 2.75 + 2.25
This tells us that one pound of methane reacts with 4 pounds of oxygen to yield 2.75 pound of carbon dioxide and 2.25 pounds of water.