 |
Combustion Process - Continued.
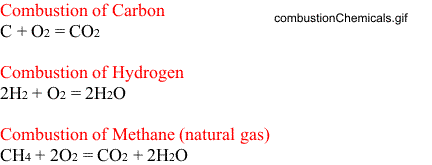
Combustion of Carbon
As noted in the above figure, for complete combustion the following reaction takes place:
One atom of carbon combines with two atoms of oxygen to produce one molecule of carbon dioxide.
We can write the same equation in terms of atomic weights.
12 + 32 = 44 (using atomic weight of entities)
Dividing all quantities by 12 we get:
12/12 + 32/12 = 44/12
We simplify the above to get:
1 + 2.67 = 3.67
In other words, one pound of carbon reacts with 2.67 pounds of oxygen to yield 3.67 pounds of carbon dioxide.