 |
Vacuum Distillation
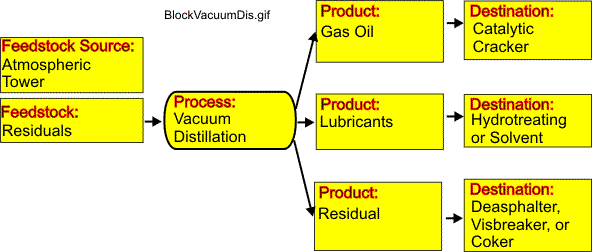
The figure provided here illustrates a block diagram for a vacuum distillation system.
Before we discuss this type of distillation, let’s review a fundamental point. A liquid is vaporized if subjected to one of two types of actions:
a. Heat it to increase its vapor pressure to the extent it exceeds the ambient pressure. In other words, heat it to its boiling point. The boiling point of a liquid depends upon the ambient pressure. This means that you need to heat the liquid to the temperature that is equal to the boiling point for the prevailing ambient pressure.
b. Reduce its ambient pressure so that the liquid can boil at a lower temperature. In a vacuum tower, we create vacuum (or, in other words, reduce pressure to a value below that of atmospheric pressure). This allows the liquid to boil at a lower temperature. Another advantage this process has is that the liquid is not subjected to higher temperatures, thus protecting sensitive chemicals from higher temperatures.